4. Performance testing – In vitro & EX VIVO models for percutaneous absorption
RaDes established a range of powerful in vitro and ex vivo models for de-risking dermal development projects and to assess the quality and performance of topical products. These models provide support through the entire lifecycle of a drug product from compound and formulation selection to bioequivalence testing and post-approval changes. High-performance analytical equipment is in place for selective and sensitive analysis.
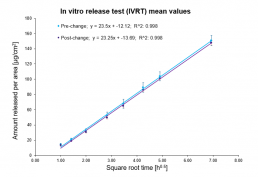
In vitro release test (IVRT) using vertical diffusion cells
- Determination of in vitro drug release rate through an artificial membrane
- Ranking and selection of formulation prototypes for dermal or transdermal application
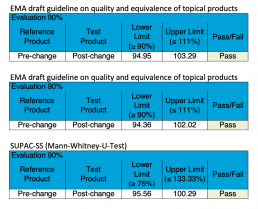
- Evaluation of equivalence of topical products for generic submissions as required according to the “EMA Draft guideline on quality and equivalence of topical products”
- Scale-up and post approval changes (according to FDA’s SUPACC SS)
- Support from initial assessment up to fully validated methods according to OECD, FDA, or EMA, including analytical method validation according ICH guidelines.
- Robotic diffusion system with dry-heat blocks and optimized stirring to obtain superior data quality. 12 parallel vertical diffusion cells in place
- For introduction and examples on IVRT see RaDes webinar recording here (YouTube).
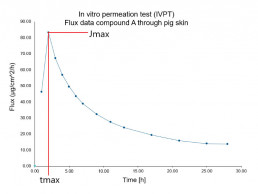
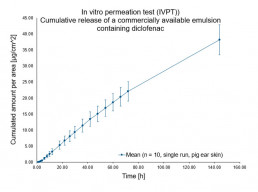
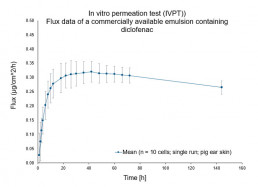
In vitro permeation test (IVPT) using vertical diffusion cells
- Evaluation of the characteristic permeation profile of the drug/ingredient(flux rate & permeated amount) through animal or human skin
- Lead compound and formulation prototype selection to de-risk clinical program
- Bioequivalence (BE) studies for topical generic development according to EMA “Draft guideline on quality and equivalence of topical products”
- Absorption studies for safety of chemicals, pesticides etc. according OECD 428
- In vitro dermal absorption studies of cosmetic / consumer products according to Scientific Committee on Consumer Safety (SCCS) guidance document
- Scale-up and post-approval changes (SUPAC SS)
- Support from initial assessment up to fully validated methods according to OECD, FDA, or EMA, including analytical method validation according to ICH guidelines.
- Investigation of distribution between epidermis and dermis (heat splitting)
- Inhouse preparation of pig ear skin samples established to use fresh pig skin in IVPT studies on demand
- Optionally, use of Strat-M® artificial, non-animal-based membrane as screening tool for skin permeation of topical formulations and for transdermal patches
- Robotic diffusion system with dry-heat blocks and optimized stirring to obtain superior data quality. 12 parallel vertical diffusion cells in place
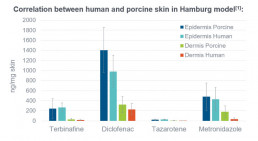
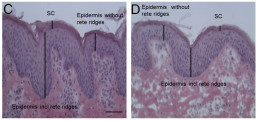
Ex vivo skin penetration model (Hamburg model)
- Skin penetration model using viable pig ear skin. Excellent correlation with human skin demonstrated
- Allows determination of cutaneous biodistribution of topically applied drugs
- Penetration profile into different skin layers. Direct determination of actives concentration at the site of action possible.
- Cost effective and without ethical implications
- In cooperation with university hospital Hamburg Eppendorf (UKE). Analytical method development, sample preparation and analysis at RaDes
- Viable model which allows determination of metabolism of actives in the skin
- See: Herbig ME, Houdek P, Gorissen S, Brandner J et al. A custom-tailored model to investigate skin penetration in porcine skin and its correlation with human skin. Eur J Pharm Biopharm, (95) 99-109, 2015
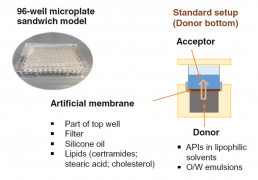
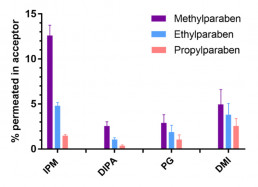
Skin Pampa
- Screening tool for dermal permeation potential
- 96-well plate format using a porous lipid-impregnated filter to mimic the stratum corneum as the main barrier for dermal absorption
- Allows comparison of topical and transdermal formulations to support ranking & selection
- RaDes investigations contributed to the understanding of the potential and limits of the model. See: Köllmer M et al. Investigation of the Compatibility of the Skin PAMPA Model with Topical Formulation and Acceptor Media Additives Using Different Assay Setups. AAPS PharmSciTech, 20(89), 2019.
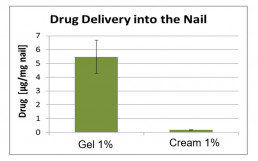
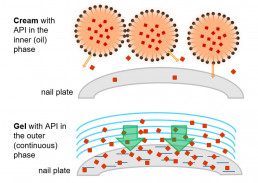
Nail penetration model
The nail is a barrier fundamentally different to the skin and requires different formulation approaches and penetration models
- Inhouse developed model using human nail clippings to determine the API amount penetrated into the nail plate
- As nail swelling facilitates penetration, formulations can also be characterized with regards to their nail swelling capacity
Your contact on this topic
Sascha Gorissen
+49 151 12986843
sascha.gorissen@rades-development.com