Internship project on rheology

Rheological properties play an important role in the design, optimization, and control of semi-solid formulations, in particular also when assessing the equivalence of products.
Important aspects are:
- What is their viscosity at low and zero shear (relevant for stability), and at high shear (relevant for spreadability, manufacturing processes, and device compatibility)?
- What are the solid- and liquid-like contributions to a material’s overall stiffness (storage and loss moduli, and complex modulus)? Does the material generally behave more like a liquid or a solid (phase angle)?
- How quickly do materials lose viscosity under shear, and how quickly do they recover (thixotropy and thixotropic recovery)?
These methods allow to describe and compare semi-solids with great precision. Such tests are also part of the regulatory requirements for rheological equivalence testing. Especially the EMA “Guideline on quality and equivalence of locally applied, locally acting cutaneous products” has particularly comprehensive and detailed requirements.
In her internship project at RaDes, Nina Frank, a pharmacist in the year of practical training from the LMU University of Munich, generated relevant data to deepen the understanding of these aspects. We thank Nina for her excellent work!
RaDes wishes Happy Holidays!

Understanding the true composition of polysorbate 20: a new publication by Rades

Understanding the true complexity of excipients like polysorbate 20 (PS20) is critical to enhancing the robustness of pharmaceutical formulations. Despite its widespread use as a stabiliser for proteins, emulsifier, and solubiliser, PS20 is far from a chemically defined substance – it is a heterogeneous mixture of thousands of species.
In this study which we performed together with Boehringer Ingelheim, we combined UPLC-MS analysis with stochastic modelling to dissect PS20 at the molecular level, delivering a full quantitative characterisation across:
- Backbone class (sorbitan, isosorbide, POE)
- Esterification degree
- Oxyethylene (OE) chain length
- Fatty acid composition
With a routine-compatible workflow using two UPLC-MS methods and a modelling step we can reveal:
- A binomial distribution governs both esterification degree and OE chain length.
- Component-level resolution across 27,775 molecular species.
- Accurate prediction of saponification and hydroxyl values, validated against certificates of analysis.
Interestingly, batch-to-batch and manufacturer-specific differences were observed in both ester distribution and HLB profiles — highlighting potential implications for emulsifying and stabilising performance. For example:
Some batches showed higher triester content, others had elevated monoester content, typically associated with better solubilisation.
This level of compositional insight open the door for:
- Improved selection and qualification of PS20 materials based on batch comparison or supplier comparison
- Deeper understanding of structure-function relationships
- More precise root cause investigations in formulation development
The link below provides you free access to the paper until Nov 7, 2025: https://authors.elsevier.com/c/1loMD3Lfa-UDn6
RaDes contributions to the skin forum 2025

The Skin Forum is one of the most important conferences on the development of topical therapeutics for experts from industry and academia.
This year’s Skin Forum on June 24 & 25 in Berlin featured a series of excellent presentations on skin biology, formulation development, (biophysical) methods for bioavailability in the skin and regulatory aspects.
RaDes was represented with a stand, a scientific poster and an invited lecture. Sascha Gorissen and Michael Herbig held a number of interesting discussions on the services RaDes offers for the development of topical products, from formulation design, aspects of analytics and stability, our in vitro performance models to specific questions on excipient analytics.
In a highly regarded lecture, Michael Herbig presented a systematic approach developed by RaDes for screening active ingredient candidates for topical application. This enables data on solubility/formulation, stability and skin penetration to be determined with just 35 mg of substance. This makes it possible to select the API candidates with the best technical developability properties. These can then be developed more quickly and cost-effectively and are more likely to lead to products with superior efficacy.
The study was also presented as a poster, which can be downloaded here.
Internship project on droplets, crystals & emulsifiers

Laurenz Beck, pharmacist in the year of practical training from the university of Greifswald just completed his internship project at RaDes. He worked on several aspects relevant to the design and characterization of semi-solid formulations.
Based on RaDes leading expertise in the analysis of complex excipients, he investigated how the actual composition of PEG-fatty alcohol ether emulsifiers affects the physical stability of emulsions. PEG-fatty alcohol ether emulsifiers (brand names such as Brij; Cremophor A; Volpo and others) are widely used. While the names suggest that they contain (mainly) a single chemical species, their true composition is typically complex with subspecies over a range of HLB values. The stated (theoretical) HLB value alone is not a sufficient guide for an informed selection of the optimal type for different purposes. Laurenz investigated the suitability of different types of these emulsifiers for challenging emulsion-type formulations, such as unusual oil phases or water phases with high ion load, and gained valuable (and sometimes surprising) insights.
In addition, he compared the determination of droplet size distribution based on manual evaluation with automated and AI-based image analysis, demonstrating the great potential of automated, AI-based analysis.
We would like to thank Laurenz for his great work based on commitment, curiosity and creativity!
Welcome Finnya!

Finnya Nienau joined RaDes in January 2025 as an Analytical Scientist. She studied Pharmaceutical Sciences in Hamburg and then joined the group of Prof. Karsten Mäder at the University of Halle for her (soon also formally completed) PhD project. In her project she developed and analyzed innovative ophthalmic formulations based on electrospinning. Using an ex vivo eye model, she demonstrated an extended residence time in the eye. Her work will be published following a pending patent application.
In her role as analytical scientist at RaDes, she is responsible for analytical method development, stability studies and in vitro performance testing such as IVRT and IVPT. With her expertise in ophthalmic formulation testing, Finnya will play a key role in the development and optimization of RaDes’ in-house ophthalmic bioavailability model.
Rades wishes Happy Holidays!

New insights on polymeric thickeners from internship project

At RaDes, we regularly offer 6-months internships for pharmacists in the year of practical training to study scientific and technical aspects of general relevance to topical formulation development and/or to explore new technologies or excipients.
For the past six months, Flemming Hansen from the University of Kiel has been systematically investigating a variety of polymeric thickeners for hydrogel formulations. In particular, he studied the compatibility with typical pharmaceutical hydrogel components such as salts, alcohols and surfactants. In addition, the suitability of polymeric thickeners for emulsion gel formulations, which are typically a more elegant and rationally designable version of the classical cream stabilized by consistency agents, was investigated.
Finally, phospholipids were explored as stabilizers for “surfactant-free” emulsion gels, some of which may also have penetration-enhancing properties.
Thank you, Flemming, for a great job and valuable insights that will be helpful in future topical development projects.
RaDes poster on innovative film-forming formulations at PBP World Conference
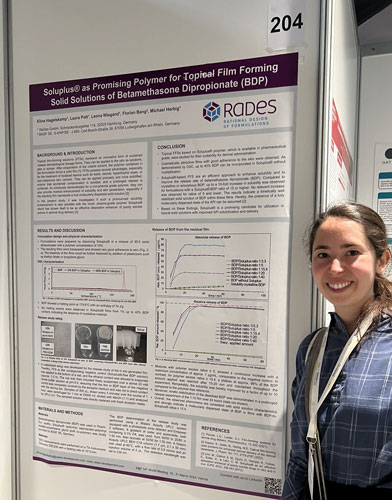
Topical film-forming solutions (FFS) are an innovative form of sustained-release dermatological dosage forms and could be particularly beneficial for the treatment of localized lesions. They can be applied precisely and quickly form a dry film, ensuring that accidental contamination is avoided and prolonged release is achieved.
RaDes has investigated FFS of betametasone dipropionate (BDP) based on the pharmaceutical grade Soluplus® polymer. Films with up to 40 % BDP in Soluplus® were produced, which are not only cosmetically appealing, but also show a significant increase in the release rate above a certain API/polymer ratio. The results of the in-vitro release study indicate good kinetic stabilization of BDP in these films and demonstrate the suitability of Soluplus® for use in topical solid solutions with improved drug solubilization and release.
Elina Hagelskamp presented the results as a poster, which can be downloaded here, at the PBP 14th World Meeting 2024 in Vienna.
If you have any questions about topical film-forming solutions or would like to learn more about how this approach can be used to enhance the release of poorly soluble active ingredients, please contact us!
Two new modern Agilent 1260 HPLC systems added to the RaDes chromatography portfolio

Analytical data quality can make a big difference in speed and success rate of liquid and semi-solid formulation development. And instruments can make a difference in data quality.
At RaDes we typically use our three Waters UPLC systems for exploratory development. They offer superior speed and the possibility of mass spec (QDa) detection in parallel with UV (PDA), which helps to accomplish even challenging analytics projects effectively.
However, also HPLC systems are an important part of our chromatography portfolio. Now we have replaced our older Agilent 1100 HPLCs by the new and more powerful 1260 Infinity II HPLC systems from Agilent.
With this setup, we have excellent instrumental equipment to support analytical method development and optimization, stability studies, and in vitro release and performance testing.
A novel LC-MS method for troubleshooting polysorbate degradation issues in biological drug formations
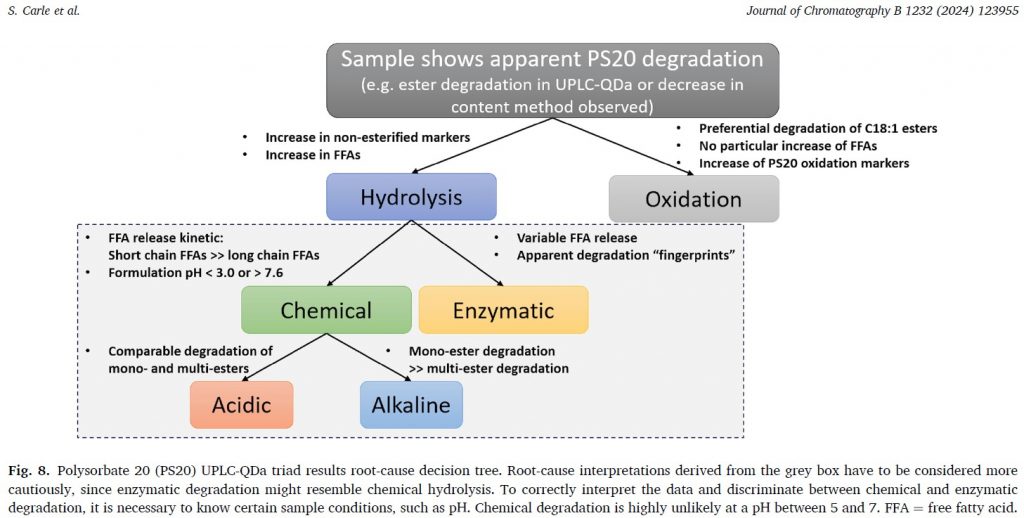
Most biologicals are stabilized by polysorbates. They have excellent stabilizing properties for injectable protein formulations and are well tolerated. However, polysorbates can degrade. This can affect their stabilizing properties and/or lead to particle formation, which is an important stability issue.
Polysorbates have a very complex composition (hundreds of subspecies) and show multiple possible degradation pathways. Therefore, troubleshooting in formulation development requires analytical methods that not only monitor decrease in the “assay”, but also allow root-cause analysis of degradation pathways.
In a recent publication by scientists from Boehringer Ingelheim together with Dirk Evers and Elina Hagelskamp from RaDes GmbH, a powerful and innovative all-in-one stability indicating analytical approach for polysorbate 20 using UPLC-QDa was presented.
The full publication can be accessed via: https://doi.org/10.1016/j.jchromb.2023.123955
The method triad includes PS20 ester stability as well as PS20 free fatty acid and PS20 oxidation marker analysis. The complete analysis can be performed from one sample preparation after protein precipitation.
The methods provide protein formulators with a new and valuable tool to accelerate and de-risk protein formulation projects through efficient root cause analysis of polysorbate stability issues.
RaDes wishes Happy Holidays!
Internship project on mucoadhesion of formulations

Whenever a drug is to be delivered to a mucous membrane such as the nasal, oral, esophageal, ophthalmic or vaginal mucosa, mucoadhesion is an important aspect of formulation design. Making formulations adhere to the mucosa helps prevent them from being washed away too quickly. Prolonging the residence time at the site of action helps to increase absorption of the drug, which improves efficacy.
There are ways to design mucoadhesive formulations, mainly by using certain polymers. Testing mucoadhesiveness is less straightforward because tissues are not readily available. Therefore, tissue-free in vitro rheological tests are very interesting to screen formulations for mucoadhesiveness. The main component of mucus is mucin, a glycoprotein with long carbohydrate chains. It is primarily the interaction of the formulation with mucin that is responsible for mucoadhesion.
During her six-month internship at RaDes, Leona Wiegand, a pharmacist in the year for practical training from the University of Kiel, studied rheological methods for investigating mucoadhesiveness. She studied the preparation of mucin solutions, the characteristics of different mucoadhesive formulations, and the potential and limitations of rheological methods for different types of formulations.
Thank you Leona for an excellent job! We wish you all the best for the future!
5 years of RaDes!

This summer, we celebrated the 5th anniversary of RaDes with a delicious dinner at the restaurant Open Kitchen at Hamburg’s Fischmarkt.
Five years is a good time to reflect on what we’ve achieved, experienced and learned.
Some facts & figures:
– We have served 42 clients from 12 different countries.
– Most of our clients are in the pharmaceutical industry. We also serve projects in cosmetics, medical devices, food supplement, novel actives and excipients, veterinary medicine, and banks/investment funds.
– Several of the pharmaceutical projects have entered preclinical and clinical phases, 7 products we’ve (co-)developed (medicinal cosmetics and medical devices) have already been launched and 4 patents on formulations we’ve developed have been filed so far.
Most importantly, we’ve built so many collaborations based on trust and mutual inspiration. Together with our clients and partners, we’ve been able to work on many meaningful and innovative projects that can have a major impact on improving people’s health and quality of life.
RaDes publication on rational design of topical semi-solid dosage forms
Advances in understanding the driving forces of skin penetration, as well as in formulation design and characterization, are enabling more efficient development of topical semi-solid formulations than in previous decades. In a recent review article by RaDes scientists, we assessed the status quo and outlined best practices for topical formulation design and characterization.
The composition of recently approved topical dermatological NCE formulations suggests that development has often followed traditional rather than rational design approaches. However, there is scientific understanding and published examples that demonstrate that rational design of topical formulations is possible. This helps to make challenging formulation projects possible and generally allows for more efficient and robust product development.
In terms of formulation characterization, we highlighted in particular rheological characterization and in vitro performance testing – two areas where significant progress has been made in recent decades.
We also outlined the key features and best practices of analytical method development for chemical stability in semi-solid formulations. This is often a critical aspect for de-risking and project success, but much less discussed in the academic literature than formulation aspects.
The article is available free of charge at: https://www.mdpi.com/1999-4923/15/7/1822
RaDes presentation on “The Benefits of Rational Design in Topical Formulation Development”
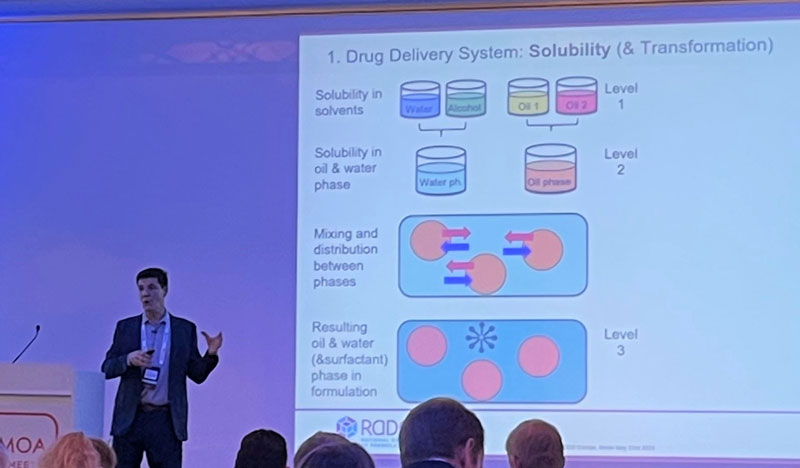
Michael Herbig from RaDes presented at the “Dermatology Drug Development Summit Europe” end of May in Berlin on “The Benefits of Rational Design in Topical Formulation Development”.
In the talk he explained how topical formulations, although often complex, can be developed rationally, reliably and efficiently. A prerequisite for this is a systematic understanding of semisolid formulations as:
I) drug delivery systems that can be described in particular in terms of solubility, saturation, distribution, and transformation
II) as physical bodies possess solid-like and liquid-like properties at the same time. Here, modern rheology offers excellent possibilities for characterization and optimization.
III) and as a “quality product” that has to meet comprehensive regulatory requirements which have to be taken into account from the beginning.
RaDes has extensive experience and scientific excellence to develop even challenging topical formulations based on “rational design”, which is more efficient and faster than traditional approaches.
In addition to the presentation, RaDes also presented a poster on the topic: Critical Excipients in Topical and Parenteral Dermatological Formulations: Novel Analytical Approaches and Risk Mitigation,” which can be downloaded here.
Rheology to improve efficacy of semi-solids
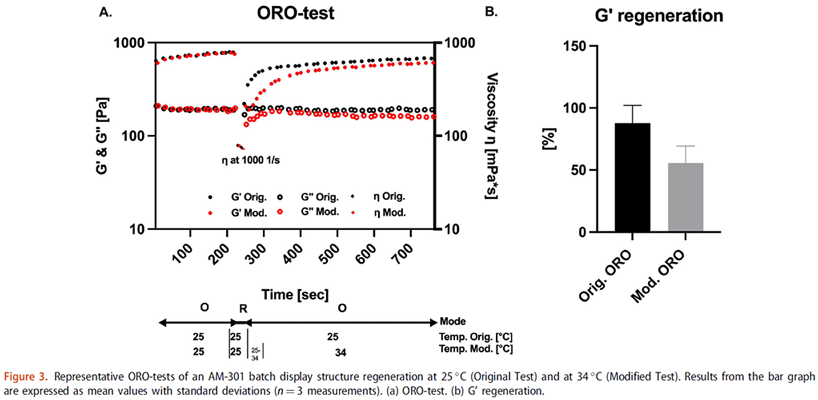
Rheological properties can markedly affect the efficacy of semisolid dosage forms.
In a recent publication with rheological investigations from RaDes, it was demonstrated that for the emulsion-type nasal spray AM-301 (now marketed as Bentrio®), specifically engineered rheological properties lead to an optimized distribution pattern and prolonged residence time in the nose. This is impressively visualized by endoscopic images of fluorescence-labelled formulations. The article is available as open access at:
DOI: 10.1080/03639045.2023.2183724
So, even if “complex modulus” and “thixotropic recovery” may sound strange, these are the parameters which can be measured by high-performance rheology and be optimized through formulation in order to improve semi-solid dosage forms. It can make emulsion or gel like-nasal sprays better sprayable and improve their residence time at the site of action in the nose, thus improving efficacy.
If you’re interested in receiving a copy of our flyer “Rheological Services to Support Formulation Development” send us an email at formulation@rades-development.com
Internship project on topical film forming solutions

Laura Pott, pharmacist in the year of practical training from the university of Münster is working on novel topical film forming solutions during her internship at RaDes.
Topical film forming solutions (FFS) have a great potential in the treatment of localized skin diseases such as warts, actinic keratoses, non-melanoma skin cancer, or keloids. They can be precisely applied (e.g. by a brush) an then quickly form a film by evaporation of the solvents. The two most important benefits from FFS are improved safety by strongly reducing the risk of contamination during and after application and enhanced skin penetration.
We previously demonstrated for a cosmetic grade film-forming polymer that skin penetration was strongly enhanced when polymer and active formed a true solid dispersion with molecularly dispersed state of the active. Laura is now exploring the pharmaceutical grade Soluplus® polymer which has excellent properties to form solid dispersions. She already identified formulation options to adjust the plasticity of the film and to optimize the viscosity of the FFS for convenient application. Now she is continuing her work with incorporation of relevant pharmaceutical actives and the characterization of the resulting films.
RADES WISHES HAPPY HOLIDAYS!

Internship project on rheological equivalence and water-free formulations
 Philipp Böhm, pharmacist in the year of practical training from the university of Heidelberg has completed his 6 months internship at RaDes. In a first part, he performed studies to assess the practicability of the criteria for rheological equivalence according to the EMA “Draft guideline on quality and equivalence of topical products”. The results, including strategic considerations how to deal with the requirements, were published in a poster presented at the “Innovations in Dermatological Sciences Conference” in September (for details see previous news posting below).
Philipp Böhm, pharmacist in the year of practical training from the university of Heidelberg has completed his 6 months internship at RaDes. In a first part, he performed studies to assess the practicability of the criteria for rheological equivalence according to the EMA “Draft guideline on quality and equivalence of topical products”. The results, including strategic considerations how to deal with the requirements, were published in a poster presented at the “Innovations in Dermatological Sciences Conference” in September (for details see previous news posting below).
In a second part, he worked on innovative, water-free formulation vehicles for incorporation of hydrolysis-prone actives or probiotics.
Many thanks to Philipp for his outstanding work!
RaDes poster on rheological equivalence testing of topical generics
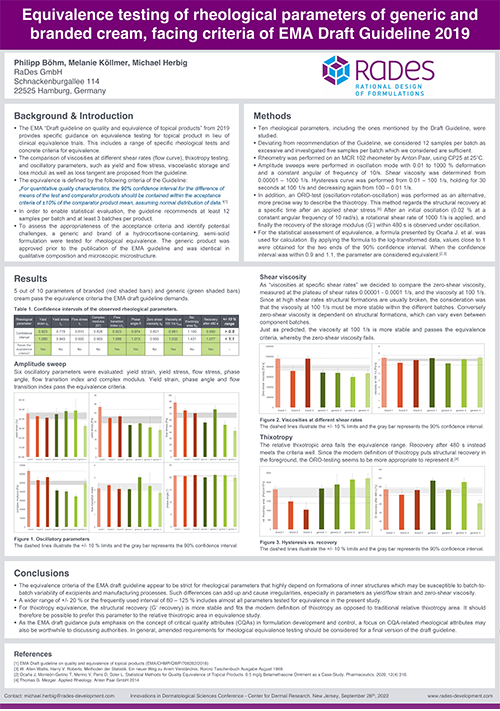
Topical dermatological generics must be characterized also for rheological equivalence to the originator. The EMA “Draft guideline on quality and equivalence of topical products” from 2019 provides specific guidance on how this should be performed.
The parameters to be investigated include viscosity values at different shear rates (viscosity curve), thixotropy testing, yield stress, as well as viscoelastic storage and loss moduli. This requires a high-performance rheometer and expertise to conduct, evaluate, and interpret the specific experiments.
RaDes presented a poster on “Equivalence testing of rheological parameters of generic and branded cream, facing criteria of EMA Draft Guideline 2019” at the “Innovations in Dermatological Sciences Conference” at the Center for Dermal Research, New Jersey, on September 28th and 29th. As we demonstrated, the proposed criteria appear very strict, especially as the variability in some parameters is high even between commercial batches of approved products. The EMA guidance is still in draft but likely to be considered ”state of the art” by the EMA. Some deviations from the proposed guideline may be possible, but should be agreed with authorities in advance based on a robust scientific rationale. Therefore, next to the rheological capabilities, a sound understanding of formulation design and of appropriate statistical evaluation are important.
The poster can be downloaded here. If you have questions on the rheological equivalence testing or if you want to learn more on how rheological characterization helps to design and optimize formulations and processes, please get in touch: michael.herbig@rades-development.com
4-year anniversary of RaDes

On a beautiful summer day in Hamburg we celebrated our 4th anniversary at RaDes GmbH with some nice drinks and food!
Many thanks to all our clients, partners and friends for the great collaboration during this time!
Internship project on Design of Experiment-optimized creams

Felix Teschke, pharmacist in the year of practical training from the university of Greifswald is completing his 6 months internship at RaDes. He dealt with the basics of the transformation of topical formulations after application. Furthermore, he has performed a Design of Experiment (DoE)-based optimization of consistency agent-containing cream formulations, especially, with respect to the ideal ratio between hydrophilic emulsifier and co-emulsifier. The results obtained are very valuable for a rational development of such creams.
Felix’s hobby is 3D printing. We have taken advantage of this to equip our labs with some self-printed, helpful tools to facilitate work processes. For example, a holder that enables particularly thorough cleaning of glass slides for sensitive microscopic examinations, or a device for microscopy slides that allows fast, safe handling of these in evaporation profile studies of topical formulations!
How rheology can be used to develop better semi-solid products & processes
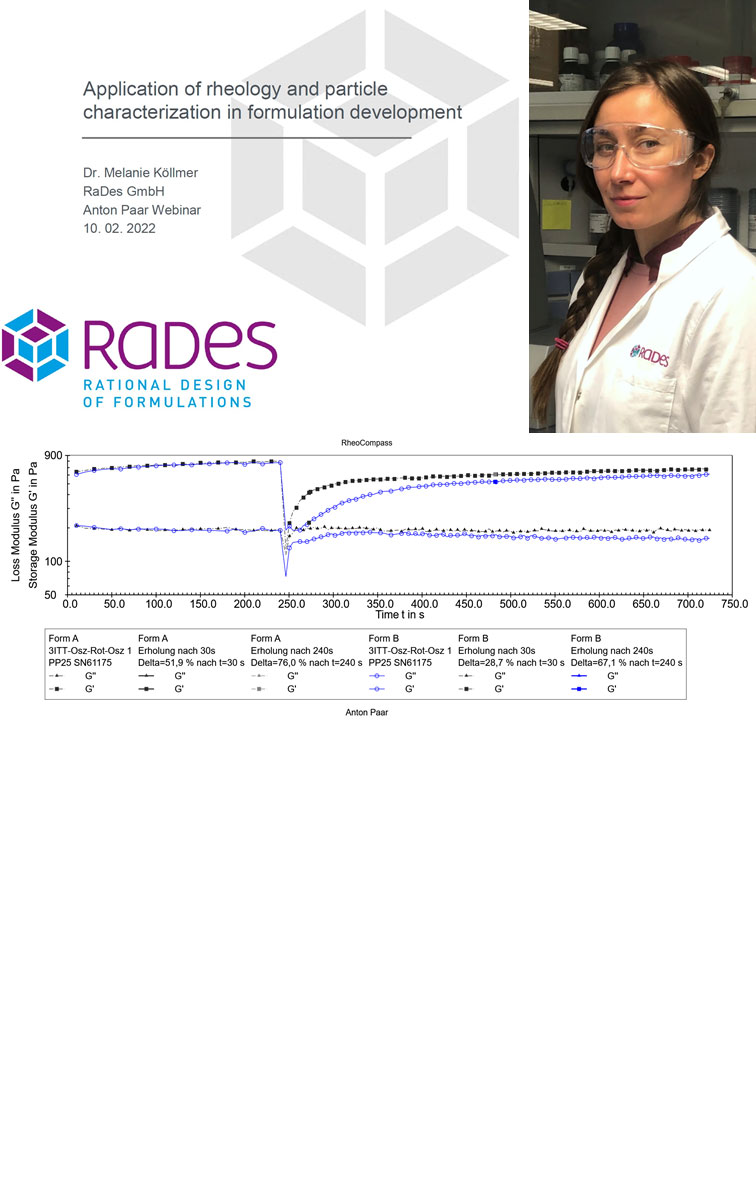
Melanie Köllmer from RaDes presented case studies on how rheology and particle size characterization helped to develop and optimize semisolid formulations in the Anton Paar webinar “A recipe for success: Combining rheology and particle characterization for pharmaceutical and cosmetic product formulation”.
Rheology and particle characterization are great tools to improve the design, process development and control of semi-solid formulations. Especially rheology offers great potential from supporting the design of attractive sensory properties to optimizing stability and diagnosing issues. In addition, increasingly comprehensive rheological characterization is requested by authorities, especially in the context of generic development projects.
Rheology is most powerful, if combined with the knowledge on formulation design and the inner structures of semisolid formulations as well as on regulatory requirements, industrial processes and constraints of quality control. Different questions require different experimental setups and evaluation of different rheological parameters.
In case you missed the presentation, you can download it here.
If you’re interested to learn more about how rheological characterization may help for the success for your project, please contact Melanie at: melanie.koellmer@rades-development.com and let’s discuss!
RaDes wishes happy holidays!

Welcome Elina!

In November 2021, Dr. Elina Hagelskamp joined RaDes as analytical scientist. Elina obtained her Master’s degree in chemistry from LMU Munich with distinction. In her PhD project in organic analytical chemistry she focused on the investigation of catalytically active molecules in the context of prebiotic chemistry and identified a class organocatalysts with potential relevance to the origin of life. In this project, she gained expertise in various applications of chromatography and mass spectrometry. Together with her sound knowledge of organic chemistry and a special interest for mathematics, she will be an important part of the analytics team at RaDes. Her focus at RaDes is analytical method development, stability investigations and in vitro release & permeation testing.
Bentrio™ – a nasal spray for protection against airborne viruses, developed with contributions from RaDes
 BentrioTM is a nasal spray which is designed to help people to protect themselves against airborne viruses and allergens. It was developed by Altamira Medica, a Swiss company, and recently got launched in Germany with more countries to follow. At RaDes, we are proud that we were able to contribute to the development of its formulation through our collaboration with Altamira.
BentrioTM is a nasal spray which is designed to help people to protect themselves against airborne viruses and allergens. It was developed by Altamira Medica, a Swiss company, and recently got launched in Germany with more countries to follow. At RaDes, we are proud that we were able to contribute to the development of its formulation through our collaboration with Altamira.
BentrioTM acts by forming a thin protective layer on the nasal mucosa. Based on physical principles, it can trap viruses such as SARS-CoV-2 and thus reduce the viral load in the nose.
Considering the challenges from the current and potential future virus variants, Bentrio can provide additional protection against Covid-19 in combination with vaccinations and general hygiene measures.
To make this possible, several interesting formulation challenges needed to be resolved: the choice of the right type and concentration of two key components, a mineral-based thickener and triglyceride, and the perfect rheological profile. The formulation liquifies under shaking to become nicely sprayable; following application to the nose, it quickly recovers viscosity to form a thin protective film on the nasal mucosa without running off (a quick “thixotropic recovery” in the words of rheology).
For more information see: www.bentrio.com
 Particle size determination of emulsions and suspensions plays an important role in formulation and process development and control. Measurement by laser diffraction offers many advantages such as the measurement of thousands of particles within seconds, a wide measurement range as well as high accuracy and reproducibility.
Particle size determination of emulsions and suspensions plays an important role in formulation and process development and control. Measurement by laser diffraction offers many advantages such as the measurement of thousands of particles within seconds, a wide measurement range as well as high accuracy and reproducibility.
However, often emulsions are more complex than just “oil droplets in water”. For example, gel phase structures formed from emulsifiers and co-emulsifiers may exist in parallel to the oil droplets. This raises the question if there are methods which are suitable of determining both species in parallel or procedures that could reliably “destroy” the gel phases to determine the primary oil droplet size only. Also, in some emulsions, oil droplets may be covered by multiple layers of emulsifiers which may or may not be subject to change during sample preparation.
In addition, the choice of the appropriate model for data processing is not always trivial: The Fraunhofer model requires only few input parameters but will lose validity for small particle sizes whereas the more universal Mie model requires more input parameters.
Hossa Bonyadi, pharmacist in the year of practical training from the university of Marburg, addresses such questions during her 6 months internship at RaDes. She performs the studies on a PSA 1090 particle sizer from Anton Paar GmbH. Her results will help to choose the appropriate sample preparation and evaluation models for different formulation types and help in the interpretation of particle size distribution data of complex emulsions.

Three years ago, at the beginning of July 2018, we started RaDes. From scratch. That means: building the labs, acquiring instruments, creating a website, considering when it was the right time to approach the first potential clients, etc. Hard work and quite a bit of uncertainty during a hot summer.
This seems so long ago now. In the meantime, we’ve established great partnerships with many clients and successfully solved a variety of development challenges from formulating actives to developing analytical methods to improving the robustness of existing products.
We’re now serving customers in Germany, Switzerland, France, UK, Austria, Slovakia and the USA. Some first products which we’ve developed already reached the market. We’re establishing a QMS, published papers, filed a patent, gave lectures and talks and continued to learn so much from collaborations and discussions. We hired new colleagues and continuously expanded our instrumentation.
Thank you to all our clients, partners, friends and, in particular, to our great team who made this possible!
A lot to celebrate – due to the pandemic, still with restricted opportunities to do so.
For now, we started with a delicious cake. Soon, the whole RaDes team will be fully vaccinated and we’ll take the opportunity to catch up with celebrating together!
For chromatography and mass spectrometry, we have acquired several instruments from Waters – a world leading manufacturer in this field.
Waters found our history and projects interesting and published a case study on us based on an extensive interview with our head of laboratory Sascha Gorissen.
Read the report with interesting insights at: Case Study RaDes – Waters
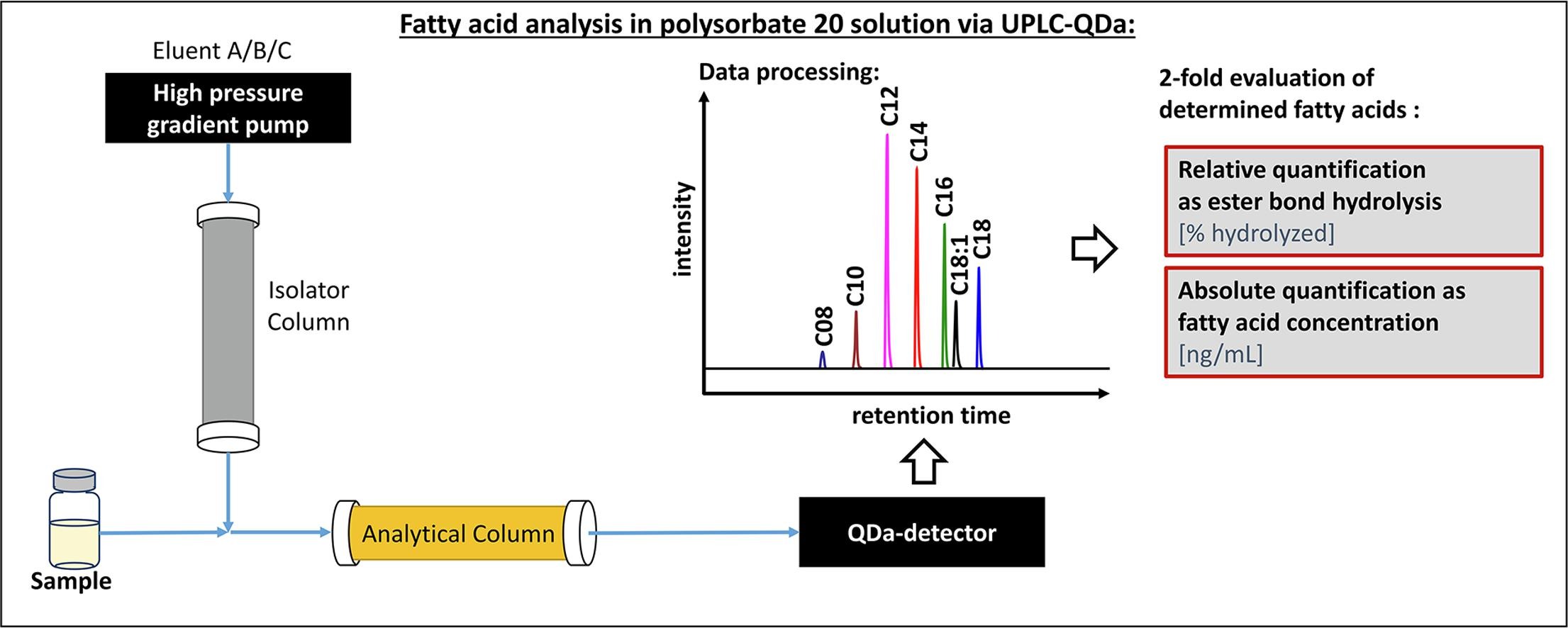
Following up on the last year’s publication on a novel quantification method for polysorbate 20, now a paper focusing on the detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics was published. Again, Dirk Evers from RaDes made essential contributions to this work.
Sensitive and robust quantification of fatty acids (FA) is essential for the monitoring, understanding and potential improvement of the stability of polysorbates in biopharmaceutical formulations.
FA may be released over time by enzymatic degradation of polysorbates caused by traces of host cell enzymes in biologics. Due to their low aqueous solubility, fatty acids may precipitate as particles which is an important concern for quality and safety.
The method presented offers several advantages for convenient monitoring of FA release: It is label-free and allows the determination of released FAs as percentage of ester bond hydrolysis and as absolute concentration. Additionally, by using an isolator column to remove trace levels of FAs present in the eluents the sensitivity of the method is improved. It was the first time in FA analysis that such a procedure was successfully employed.
For more information, see the full publication at https://www.sciencedirect.com/science/article/pii/S1570023221001975 or contact us at info@rades-development.com

For what we do at RaDes, the Waters UPLC system equipped with eλ-PDA (UV/VIS) plus QDa (single mass) detectors proofed to be extremely useful. Therefore, to support our growth, we just got our third one installed!
The system permits us to speed up method development and the rapid analysis also of larger quantities of stability and IVRT samples. Thanks to the QDa mass spec detector, it allows the detection of also poorly UV-active compounds with high sensitivity and the identification of co-eluting compounds.
An instructive example of how this adds value to challenging analytical projects, you may have a look at the recent publication on identification and quantification of the complex mixture of subspecies in polysorbate 20: https://doi.org/10.1016/j.jchromb.2020.122287.
Many thanks to Waters for the continued great cooperation!

Der „Jugend forscht“-Wettbewerb ist eine großartige Möglichkeit für Jugendliche, ihr Talent in einem selbstgewählten wissenschaftlichen Projekt auszuprobieren und weiterzuentwickeln. Zum dritten Mal in den zweieinhalb Jahren seit Bestehen unterstützte RaDes den „Jugend forscht“ Regionalwettbewerb Hamburg Volkspark durch zwei Juroren im Bereich „Chemie“ (https://www.hamburg.jugend-forscht.de).
In diesem Jahr haben Melanie Köllmer und Dirk Evers eine Reihe spannender Projekte von jungen naturwissenschaftlichen Talenten begutachtet – Pandemie-bedingt in einem reinen Online-Format. Zum zweiten Mal hat die RaDes GmbH dabei ein Schnupperpraktikum in den RaDes Laboratorien als Sonderpreis für ein herausragendes Projekt vergeben. Wir freuen uns, auf diesem Weg einen Beitrag zur Förderung und Begeisterung junger Menschen für die Forschung & Entwicklung zu leisten.

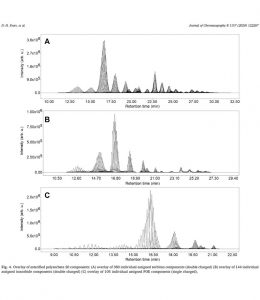
Polysorbates are used in around 70% of all biopharmaceutical formulations as generally safe and effective stabilizers. However, in recent years, the risk of particle formation in parenterals caused by chemical or enzymatic degradation of polysorbates gained attention.
Polysorbates are complex mixtures; theoretically, the number of possible subspecies may be infinite. To enable the mitigation of risks, powerful and reliable analytical methods are necessary which allow separation, identification, and quantification of the subspecies. This then enables a mechanistic understanding of degradation pathways as basis for improved product safety.
We are pleased that now, with essential contributions of Dirk Evers from RaDes, a novel method for the selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations was published. Using a modern Waters UPLC-H Class with selective QDa detector, 676 subspecies could be assigned. For a representative selection of 41 markers a quantification method was developed and validated.
For the full article see: https://www.sciencedirect.com/science/article/pii/S1570023220300143?via%3Dihub
Contact: info@rades-development.com

As of mid-August 2020, Mandy Rosenberger, joined our team as chemical laboratory technician. She has several years of practical work experience in the laboratory and is familiar with a wide range of analytical methods. Her main focus at RaDes is sample preparation of semi-solid formulations, chromatography with UV/Vis and mass spectrometric detection, as well as support in the manufacturing of formulations.
We are very happy that Mandy is now part of our team!
For two months now, life and work have been strongly influenced by the effects of the Corona pandemic.
At RaDes, we took early action and prepared ourselves. Anticipating and mitigating risks is a well-established practice for us. We are stocked with important materials (eluents, excipients) and hold our meetings virtually to avoid non-essential contacts.
An important finding is that it has paid off that we have invested in a powerful IT infrastructure from the very beginning: every employee has a notebook, data and analysis instruments can be securely accessed from home. Our laboratory operations continue in accordance with official regulations and a “backup team” working from home is in place to ensure business continuity and to be reliably available for our customers.
We take the situation seriously in order to protect the health of our employees and to act far-sighted and socially responsibly. We also do not lose sight of what was important before “Corona” and is crucial for the future: many things that make up good development work will continue to exist. Some things will change. The use of modern technology and digitalisation is changing from “nice to have” to a must have in many areas.
Finally, we would like to take this opportunity to thank our customers, suppliers and business partners. During this time, we have had many positive experiences of friendly and constructive cooperation and commitment to move things forward together in spite of obstacles.

As of May, Manasse Feichtinger started a six-month internship at RaDes as part of his compulsory training as pharmacist. Manasse studied pharmaceutical sciences at the university of Marburg; in addition to that, he is a trained laboratory technician with experience in the analysis of semi-solid formulations.
At RaDes, Manasse is going to investigate rheological characteristics of semi-solid excipients and formulations, including oscillatory studies that allow deeper insights to the inner structure of materials, as well as thermo-rheology.
A particular focus is to what extent materials from different suppliers, which all comply with pharmacopeial specifications, show rheological differences that may be relevant for stability, process development, and performance. In addition, it will be investigated how rheological features of pure structure-giving excipients relate to their performance after incorporation in a formulation.
In RaDes’ second year of existence, this is our first student project. As we are committed to the development of talents, innovation, and the ambition to understand semi-solid formulations more and more systematically, we expect that more will follow!

Lack of control over variability factors can compromise data quality of in vitro release testing and cause ambiguity in the assessment of skin permeation.
We are proud of having implemented Germany’s first Phoenix™ RDS (Robotic Diffusion Station) with dry heat diffusion cells and being able to offer the benefits of this technology to our customers.
Its advantages versus the traditional technology of in vitro permeation testing include a significantly better temperature control provided by the Phoenix dry heat system as compared to circulating water baths systems. As diffusion is a function of temperature, this improves data quality. Similarly, a superior control of mixing speed is achieved. In addition, reduced maintenance and faster setup making it more economical.
Many thanks to Bruna Lousada from Teledyne Hansen Research and Severin Schweisthal & Antony Wozniak from Cole Parmer for the great collaboration and support!
www.hansonresearch.com/diffusion-testing/phoenix-dry-heat-systems
 We are very pleased that Dr. Petra Lohmann joined the RaDes team at the beginning of January 2020 as head of quality assurance.
We are very pleased that Dr. Petra Lohmann joined the RaDes team at the beginning of January 2020 as head of quality assurance.
Petra Lohmann has more than 20 years of experience in quality control and quality assurance in responsible positions, which makes her extensively familiar with quality requirements for pharmaceuticals, medical devices, cosmetics and biocides.
She is responsible for the development and maintenance of the quality management system at RaDes and offers consulting services and expert statements in the field of quality management.
Further information is available at: https://rades-development.com/en/about-rades/team/dr-petra-lohmann/
RaDes war eingeladen, sich auf der „Ins Netz gegangen“-Veranstaltung von Life Science Nord zu präsentieren. Die einzige Regel des Events, das am 27.11.19 in Lübeck stattfand, war: die Präsentation darf maximal 6 Minuten dauern. Michael Herbig, Geschäftsführer von RaDes, hat die Firma in 5 Minuten und 42 Sekunden vorgestellt und sich über die interessanten Diskussionen mit anderen Netzwerkmitgliedern im Anschluss gefreut!
Unter “RaDes bei Life Science Nord” finden Sie unsere Präsentation, für die Sie sich so viel Zeit nehmen können, wie Sie möchten.
On 20.09.2019 our employee Janek Giebel was awarded by the Chamber of Industry and Commerce of Lübeck for his outstanding performance in the final examination as a chemical laboratory technician. We congratulate him very much and are happy that he enriches our team with his excellent work!
 On 23 – 24 September Melanie Köllmer and Michael Herbig attended the Skin Forum in Reims, France. They enjoyed the scientific program with many interesting and relevant contributions and the inspiring discussions with other participants. Also, our poster “Differences in shear viscosities in the non-plateau region of the viscosity curve and implications for rheological method development and release testing of semisolid products” was well received and led to interesting discussions. For more information on services we offer around rheological characterization of formulation have look at our attached flyer.
On 23 – 24 September Melanie Köllmer and Michael Herbig attended the Skin Forum in Reims, France. They enjoyed the scientific program with many interesting and relevant contributions and the inspiring discussions with other participants. Also, our poster “Differences in shear viscosities in the non-plateau region of the viscosity curve and implications for rheological method development and release testing of semisolid products” was well received and led to interesting discussions. For more information on services we offer around rheological characterization of formulation have look at our attached flyer.

As of July, Janek Giebel has joined the RaDes team! He very successfully finished his apprenticeship to become a chemical laboratory technician in June 2019. During his training he already collected 3 years of experience in the analysis of semi-solid formulations.
Janek will mainly support us in analytical work (HPLC, UPLC, MS) as well as in galenic development and testing of liquid and semi-solid products.
We are happy that Janek is part of our team!

After a successful first year we have extended our modern analytical equipment with an additional Waters ACQUITY™ UPLC™- H-Class with PDA & QDa (single mass spectrometer) detectors. The new system is also equipped with a solvent selection valve (6 mobile phases) and a column manager (up to 4 columns) and is therefore ideally suited for method development as well as for the analysis of large sample quantities.
All our HPLC and UPLC™ instruments are operated with the latest version of the chromatography data system Empower™ 3 (Service Release 3) from Waters GmbH to ensure high quality data management.
The new equipment allows us to further increase our capacity and flexibility in handling customer projects.
At the beginning of July 2018, the history of RaDes began with the set-up of the laboratories and the creation of the website. In September the founding team was complete, in November the laboratories were fully operational. And after one year we are an established, growing development service provider!
We are grateful for the many positive experiences and collaborations and would like to thank our customers, partners, friends and look forward to further collaborations!

In the first months of this year, three research papers were published by RaDes scientists. These address different aspects with high practical relevance for the systematic development of liquid and semi-solid formulations:
The first paper published was a systematic evaluation of the Skin PAMPA model (‘Investigation of the Compatibility of the Skin PAMPA Model with Topical Formulation and Acceptor Media Additives Using Different Assay Setups’). In this, the general compatibility of the model with non-aqueous formulation systems was demonstrated and solvent concentrations above which there is a risk of extraction of lipids from the membrane were established. In addition, the potentials and limitations of a modified setup placing the donor on top were described.
DOI: https://doi.org/10.1208/s12249-019-1305-3
Furthermore, the influence of the aqueous concentration of betamethasone dipropionate on its stability in emulsion gels was investigated. It could be shown that with increasing emulsifier concentration more active substance was distributed into the water phase which causes increased degradation. The methods and insights pave the way to formulation options to stabilize hydrolysis-prone actives by “entrapping” them in the oil phase. DOI
DOI: https://doi.org/10.1016/j.ijpharm.2019.02.044
In order to determine the emulsifier content in the water phase, a new method for quantifying polysorbate 80 was developed. This means that polysorbate 80 can also be determined in concentrations far below the critical micelle formation concentration. Using a DoE approach, it was also possible to determine how the oleic acid contained in this highly complex mixture can be reliably determined in the presence of isomeric C18:1 acids.
DOI: https://doi.org/10.1016/j.chroma.2019.04.015
If you have any questions or comments or if you see potential how our methods and insights can be helpful in your projects, please do not hesitate to contact us: info@rades-development.com or +49-40-57004180

Julia Puschmann of RaDes GmbH was awarded the first prize of the “Hans Christan Korting-Nachwuchspreis für Dermopharmazie” at the annual conference of the Society for Dermopharmacy and had the opportunity to present her work in two short talks. We congratulate Julia on her award!
She received it for her poster entitled “How does the emulsifier concentration affect API stability in emulsion gels and skin penetration?”. It demonstrates that it is possible to stabilize hydrolysis-prone actives in aqueous creams if the active is lipophilic and you use the right formulation technology! Using the active betamethasone dipropionate, she elucidated the underlying mechanisms, such as the influence of emulsifier concentration and the correlation of degradation with increasing free drug concentration in the aqueous phase.
In addition, she demonstrated that the skin penetration, tested on the pig ear with the Hamburg model (https://doi.org/10.1016/j.ejpb.2015.03.030), was further influenced by a changed saturation of the formulation upon addition of co-solvents.
Meanwhile, her corresponding paper was also published: (https://doi.org/10.1016/j.ijpharm.2019.02.044).
If you have any questions or comments, don’t hesitate to contact us: info@rades-development.com
Emulsions are important systems in drug delivery, especially for topical application,  but also orally and parenterally. The APV workshop on 01 to 02 April 2019 will provide a comprehensive insight, including formulation, characterization and processes.
but also orally and parenterally. The APV workshop on 01 to 02 April 2019 will provide a comprehensive insight, including formulation, characterization and processes.
Michael Herbig of RaDes will give a presentation on: ”Cream 2.0“ – Enhancing scope, performance and robustness of emulsions through rational design”. He is presenting new insights in distribution phenomena in emulsions and their effects on stability and performance, on effects of emulsifiers, consistency adjusting systems and approaches to modify skin penetration.
Find the program details here: https://www.apv-mainz.de/fileadmin/dateiablage/apv-mainz/Seminare/oeffentllich/6767_Emulsions_experts.pdf
If you’re interested in further information, don’t hesitate to contact us: info@rades-development.com.

With the implementation of the rheometer MCR 102 by Anton Paar GmbH we have added a new tool for formulation characterization to our equipment list. With this instrument we can perform both oscillatory and rotational measurements of semisolids and liquids. By variation of the chosen system (plate/plate, cone/plate, cylinder/beaker) we can obtain information on structure and behavior under stress of different formulation types.
Oscillation rheology allows prognosis for long-term stability and behavior under stress e.g. pumping into the storage vessel. Additionally, the influence of stress on the formulation can be measured. Using special methods, also the recovery of the structure after stress application can be shown. Does the formulation reach its structure before the stress application or is the formulation changed due to the destruction of the inner structure? Furthermore, the impact of varying concentrations, batches and suppliers of excipients can be compared to collect data on critical quality attributes e.g. the behavior under thermal stress.
Various formulations regarding type (emulsions, creams, ointments etc.), excipients (critical quality attributes) and storage conditions can be compared to evaluate the best possible prototype for your development and to support the trouble shooting of existing products.
If you have any questions, don’t hesitate to contact us: info@rades-development.com

Since end of October, a Waters ACQUITY™ UPLC™ H-Class PLUS with PDA eλ (UV/VIS) & QDa (single mass spectrometer) detectors expands the analytical equipment at RaDes GmbH.
We can cover a broad range of customer needs – from method development using Quality by Design, the transfer of analytical methods between HPLC and UPLC™ technology, up to the fast and reliable analysis of a large sample size.
What do these detectors stand for?
PDA eλ: highly sensitive photodiode array UV/VIS detector with an extended wavelength range between 190 nm to 800 nm to receive spectral information up to the visible range (e.g. to investigate discolorations) and for a robust quantification of compounds over a wide linear range.
QDa: Mass spectrometer (size of a UV/VIS detector) for peak tracking, quantification of compounds at a low concentration level or with a low UV absorption, assessment of co-elution.
The system contains next a solvent selection valve (six additional solvents) a column manager (four columns) to significantly save time and resources during method development.
All our HPLC and UPLC™ instruments are connected to the latest version of Empower™ 3 (Service Release 3) of Waters GmbH to ensure high-quality data management.
Particular benefits for our customers include:
1) Analysis of poor UV-absorbing molecules such as macrolides and peptides – a trend in dermatology.
2) Analysis of low dose formulations – another trend in dermatology.
3) Faster and better method development for classical HPLC:
- The method is developed using a DoE approach with a UPLC™. If necessary, co-eluting peaks are separated by mass spectrometry using the QDa detector and can therefore be precisely evaluated.
- The optimum conditions are selected from the design space considering resolution, run time and robustness.
- in silico transformation into a HPLC method using DoE.
- experimental confirmation on classical HPLC.
The preservation of creams and emulsions is often challenging. On the one hand, dermal drugs must be preserved in accordance with pharmacopoeia regulations in order to actively reduce microorganisms introduced. On the other hand, the number and concentration of the preservatives used should be kept as low as possible in order to optimize the compatibility of the formulation. Generally, depending on the product category and region (EU / USA), there are also maximum concentrations for the use of preservatives. Furthermore, a number of potential incompatibilities of preservatives with other formulation ingredients or primary packaging should be considered.
In the recently published study “Correlation of antimicrobial effects of phenoxyethanol with its free concentration in the water phase of o / w-emulsion gels.” (Puschmann et al., Eur J Pharm Biopharm (131) 152-161, 2018, www.sciencedirect.com) scientists from RaDes GmbH in collaboration with the Pharmaceutical Technology of the TU Braunschweig were able to gain new insights with high practical relevance for the optimization of preservation systems in emulsions.
We were able to establish a new method to accurately determine the concentration of the widely used phenoxyethanol preservative in the oil and water phases. In addition, it was possible to distinguish between free and micellar bound preservative within the water phase. As we expected, antimicrobial efficacy does not depend on the total concentration of the preservative but on the free concentration in the water phase. This in turn can be significantly influenced by the composition of the oil phase, by co-solvents in the water phase and by emulsifier type and concentration.
Our methods and insights make it possible to develop preservative systems faster and more robustly and to optimize the overall system in terms of antimicrobial performance, technical robustness and skin tolerance. Last but not least, based on this, the number of germ load tests necessary in the development phase can be purposefully reduced, thereby saving time and costs. This approach can also contribute significantly to the development of new, optimized preservatives.
For chromatography and mass spectrometry, we have acquired several instruments from Waters – a world leading manufacturer in this field.
Waters found our history and projects interesting and published a case study on us based on an extensive interview with our head of laboratory Sascha Gorissen.
Read the report with interesting insights at: Case Study RaDes – Waters
For chromatography and mass spectrometry, we have acquired several instruments from Waters – a world leading manufacturer in this field.
Waters found our history and projects interesting and published a case study on us based on an extensive interview with our head of laboratory Sascha Gorissen.
Read the report with interesting insights at: Case Study RaDes – Waters
For our growing team we are looking for an analytical scientist with focus on chromatography.
Awaiting you: versatile, challenging R&D projects, a friendly, dedicated, professional team and opportunities to have an impact and develop yourself further!
Interested: Have a look at: Ausschreibung Wissenschaftler (m/w/d) Analytik
Following up on the last year’s publication on a novel quantification method for polysorbate 20, now a paper focusing on the detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics was published. Again, Dirk Evers from RaDes made essential contributions to this work.
Sensitive and robust quantification of fatty acids (FA) is essential for the monitoring, understanding and potential improvement of the stability of polysorbates in biopharmaceutical formulations.
FA may be released over time by enzymatic degradation of polysorbates caused by traces of host cell enzymes in biologics. Due to their low aqueous solubility, fatty acids may precipitate as particles which is an important concern for quality and safety.
The method presented offers several advantages for convenient monitoring of FA release: It is label-free and allows the determination of released FAs as percentage of ester bond hydrolysis and as absolute concentration. Additionally, by using an isolator column to remove trace levels of FAs present in the eluents the sensitivity of the method is improved. It was the first time in FA analysis that such a procedure was successfully employed.
For more information, see the full publication at https://www.sciencedirect.com/science/article/pii/S1570023221001975 or contact us at info@rades-development.com


For what we do at RaDes, the Waters UPLC system equipped with eλ-PDA (UV/VIS) plus QDa (single mass) detectors proofed to be extremely useful. Therefore, to support our growth, we just got our third one installed!
The system permits us to speed up method development and the rapid analysis also of larger quantities of stability and IVRT samples. Thanks to the QDa mass spec detector, it allows the detection of also poorly UV-active compounds with high sensitivity and the identification of co-eluting compounds.
An instructive example of how this adds value to challenging analytical projects, you may have a look at the recent publication on identification and quantification of the complex mixture of subspecies in polysorbate 20: https://doi.org/10.1016/j.jchromb.2020.122287.
Many thanks to Waters for the continued great cooperation!

Der „Jugend forscht“-Wettbewerb ist eine großartige Möglichkeit für Jugendliche, ihr Talent in einem selbstgewählten wissenschaftlichen Projekt auszuprobieren und weiterzuentwickeln. Zum dritten Mal in den zweieinhalb Jahren seit Bestehen unterstützte RaDes den „Jugend forscht“ Regionalwettbewerb Hamburg Volkspark durch zwei Juroren im Bereich „Chemie“ (https://www.hamburg.jugend-forscht.de).
In diesem Jahr haben Melanie Köllmer und Dirk Evers eine Reihe spannender Projekte von jungen naturwissenschaftlichen Talenten begutachtet – Pandemie-bedingt in einem reinen Online-Format. Zum zweiten Mal hat die RaDes GmbH dabei ein Schnupperpraktikum in den RaDes Laboratorien als Sonderpreis für ein herausragendes Projekt vergeben. Wir freuen uns, auf diesem Weg einen Beitrag zur Förderung und Begeisterung junger Menschen für die Forschung & Entwicklung zu leisten.


Polysorbates are used in around 70% of all biopharmaceutical formulations as generally safe and effective stabilizers. However, in recent years, the risk of particle formation in parenterals caused by chemical or enzymatic degradation of polysorbates gained attention.
Polysorbates are complex mixtures; theoretically, the number of possible subspecies may be infinite. To enable the mitigation of risks, powerful and reliable analytical methods are necessary which allow separation, identification, and quantification of the subspecies. This then enables a mechanistic understanding of degradation pathways as basis for improved product safety.
We are pleased that now, with essential contributions of Dirk Evers from RaDes, a novel method for the selective marker-based quantification of polysorbate 20 in biopharmaceutical formulations was published. Using a modern Waters UPLC-H Class with selective QDa detector, 676 subspecies could be assigned. For a representative selection of 41 markers a quantification method was developed and validated.
For the full article see: https://www.sciencedirect.com/science/article/pii/S1570023220300143?via%3Dihub
Contact: info@rades-development.com

As of mid-August 2020, Mandy Rosenberger, joined our team as chemical laboratory technician. She has several years of practical work experience in the laboratory and is familiar with a wide range of analytical methods. Her main focus at RaDes is sample preparation of semi-solid formulations, chromatography with UV/Vis and mass spectrometric detection, as well as support in the manufacturing of formulations.
We are very happy that Mandy is now part of our team!
For two months now, life and work have been strongly influenced by the effects of the Corona pandemic.
At RaDes, we took early action and prepared ourselves. Anticipating and mitigating risks is a well-established practice for us. We are stocked with important materials (eluents, excipients) and hold our meetings virtually to avoid non-essential contacts.
An important finding is that it has paid off that we have invested in a powerful IT infrastructure from the very beginning: every employee has a notebook, data and analysis instruments can be securely accessed from home. Our laboratory operations continue in accordance with official regulations and a “backup team” working from home is in place to ensure business continuity and to be reliably available for our customers.
We take the situation seriously in order to protect the health of our employees and to act far-sighted and socially responsibly. We also do not lose sight of what was important before “Corona” and is crucial for the future: many things that make up good development work will continue to exist. Some things will change. The use of modern technology and digitalisation is changing from “nice to have” to a must have in many areas.
Finally, we would like to take this opportunity to thank our customers, suppliers and business partners. During this time, we have had many positive experiences of friendly and constructive cooperation and commitment to move things forward together in spite of obstacles.

As of May, Manasse Feichtinger started a six-month internship at RaDes as part of his compulsory training as pharmacist. Manasse studied pharmaceutical sciences at the university of Marburg; in addition to that, he is a trained laboratory technician with experience in the analysis of semi-solid formulations.
At RaDes, Manasse is going to investigate rheological characteristics of semi-solid excipients and formulations, including oscillatory studies that allow deeper insights to the inner structure of materials, as well as thermo-rheology.
A particular focus is to what extent materials from different suppliers, which all comply with pharmacopeial specifications, show rheological differences that may be relevant for stability, process development, and performance. In addition, it will be investigated how rheological features of pure structure-giving excipients relate to their performance after incorporation in a formulation.
In RaDes’ second year of existence, this is our first student project. As we are committed to the development of talents, innovation, and the ambition to understand semi-solid formulations more and more systematically, we expect that more will follow!

Lack of control over variability factors can compromise data quality of in vitro release testing and cause ambiguity in the assessment of skin permeation.
We are proud of having implemented Germany’s first Phoenix™ RDS (Robotic Diffusion Station) with dry heat diffusion cells and being able to offer the benefits of this technology to our customers.
Its advantages versus the traditional technology of in vitro permeation testing include a significantly better temperature control provided by the Phoenix dry heat system as compared to circulating water baths systems. As diffusion is a function of temperature, this improves data quality. Similarly, a superior control of mixing speed is achieved. In addition, reduced maintenance and faster setup making it more economical.
Many thanks to Bruna Lousada from Teledyne Hansen Research and Severin Schweisthal & Antony Wozniak from Cole Parmer for the great collaboration and support!
www.hansonresearch.com/diffusion-testing/phoenix-dry-heat-systems
 We are very pleased that Dr. Petra Lohmann joined the RaDes team at the beginning of January 2020 as head of quality assurance.
We are very pleased that Dr. Petra Lohmann joined the RaDes team at the beginning of January 2020 as head of quality assurance.
Petra Lohmann has more than 20 years of experience in quality control and quality assurance in responsible positions, which makes her extensively familiar with quality requirements for pharmaceuticals, medical devices, cosmetics and biocides.
She is responsible for the development and maintenance of the quality management system at RaDes and offers consulting services and expert statements in the field of quality management.
Further information is available at: https://rades-development.com/en/about-rades/team/dr-petra-lohmann/
RaDes war eingeladen, sich auf der „Ins Netz gegangen“-Veranstaltung von Life Science Nord zu präsentieren. Die einzige Regel des Events, das am 27.11.19 in Lübeck stattfand, war: die Präsentation darf maximal 6 Minuten dauern. Michael Herbig, Geschäftsführer von RaDes, hat die Firma in 5 Minuten und 42 Sekunden vorgestellt und sich über die interessanten Diskussionen mit anderen Netzwerkmitgliedern im Anschluss gefreut!
Unter “RaDes bei Life Science Nord” finden Sie unsere Präsentation, für die Sie sich so viel Zeit nehmen können, wie Sie möchten.
On 20.09.2019 our employee Janek Giebel was awarded by the Chamber of Industry and Commerce of Lübeck for his outstanding performance in the final examination as a chemical laboratory technician. We congratulate him very much and are happy that he enriches our team with his excellent work!
 On 23 – 24 September Melanie Köllmer and Michael Herbig attended the Skin Forum in Reims, France. They enjoyed the scientific program with many interesting and relevant contributions and the inspiring discussions with other participants. Also, our poster “Differences in shear viscosities in the non-plateau region of the viscosity curve and implications for rheological method development and release testing of semisolid products” was well received and led to interesting discussions. For more information on services we offer around rheological characterization of formulation have look at our attached flyer.
On 23 – 24 September Melanie Köllmer and Michael Herbig attended the Skin Forum in Reims, France. They enjoyed the scientific program with many interesting and relevant contributions and the inspiring discussions with other participants. Also, our poster “Differences in shear viscosities in the non-plateau region of the viscosity curve and implications for rheological method development and release testing of semisolid products” was well received and led to interesting discussions. For more information on services we offer around rheological characterization of formulation have look at our attached flyer.

As of July, Janek Giebel has joined the RaDes team! He very successfully finished his apprenticeship to become a chemical laboratory technician in June 2019. During his training he already collected 3 years of experience in the analysis of semi-solid formulations.
Janek will mainly support us in analytical work (HPLC, UPLC, MS) as well as in galenic development and testing of liquid and semi-solid products.
We are happy that Janek is part of our team!

After a successful first year we have extended our modern analytical equipment with an additional Waters ACQUITY™ UPLC™- H-Class with PDA & QDa (single mass spectrometer) detectors. The new system is also equipped with a solvent selection valve (6 mobile phases) and a column manager (up to 4 columns) and is therefore ideally suited for method development as well as for the analysis of large sample quantities.
All our HPLC and UPLC™ instruments are operated with the latest version of the chromatography data system Empower™ 3 (Service Release 3) from Waters GmbH to ensure high quality data management.
The new equipment allows us to further increase our capacity and flexibility in handling customer projects.
At the beginning of July 2018, the history of RaDes began with the set-up of the laboratories and the creation of the website. In September the founding team was complete, in November the laboratories were fully operational. And after one year we are an established, growing development service provider!
We are grateful for the many positive experiences and collaborations and would like to thank our customers, partners, friends and look forward to further collaborations!

In the first months of this year, three research papers were published by RaDes scientists. These address different aspects with high practical relevance for the systematic development of liquid and semi-solid formulations:
The first paper published was a systematic evaluation of the Skin PAMPA model (‘Investigation of the Compatibility of the Skin PAMPA Model with Topical Formulation and Acceptor Media Additives Using Different Assay Setups’). In this, the general compatibility of the model with non-aqueous formulation systems was demonstrated and solvent concentrations above which there is a risk of extraction of lipids from the membrane were established. In addition, the potentials and limitations of a modified setup placing the donor on top were described.
DOI: https://doi.org/10.1208/s12249-019-1305-3
Furthermore, the influence of the aqueous concentration of betamethasone dipropionate on its stability in emulsion gels was investigated. It could be shown that with increasing emulsifier concentration more active substance was distributed into the water phase which causes increased degradation. The methods and insights pave the way to formulation options to stabilize hydrolysis-prone actives by “entrapping” them in the oil phase. DOI
DOI: https://doi.org/10.1016/j.ijpharm.2019.02.044
In order to determine the emulsifier content in the water phase, a new method for quantifying polysorbate 80 was developed. This means that polysorbate 80 can also be determined in concentrations far below the critical micelle formation concentration. Using a DoE approach, it was also possible to determine how the oleic acid contained in this highly complex mixture can be reliably determined in the presence of isomeric C18:1 acids.
DOI: https://doi.org/10.1016/j.chroma.2019.04.015
If you have any questions or comments or if you see potential how our methods and insights can be helpful in your projects, please do not hesitate to contact us: info@rades-development.com or +49-40-57004180

Julia Puschmann of RaDes GmbH was awarded the first prize of the “Hans Christan Korting-Nachwuchspreis für Dermopharmazie” at the annual conference of the Society for Dermopharmacy and had the opportunity to present her work in two short talks. We congratulate Julia on her award!
She received it for her poster entitled “How does the emulsifier concentration affect API stability in emulsion gels and skin penetration?”. It demonstrates that it is possible to stabilize hydrolysis-prone actives in aqueous creams if the active is lipophilic and you use the right formulation technology! Using the active betamethasone dipropionate, she elucidated the underlying mechanisms, such as the influence of emulsifier concentration and the correlation of degradation with increasing free drug concentration in the aqueous phase.
In addition, she demonstrated that the skin penetration, tested on the pig ear with the Hamburg model (https://doi.org/10.1016/j.ejpb.2015.03.030), was further influenced by a changed saturation of the formulation upon addition of co-solvents.
Meanwhile, her corresponding paper was also published: (https://doi.org/10.1016/j.ijpharm.2019.02.044).
If you have any questions or comments, don’t hesitate to contact us: info@rades-development.com
Emulsions are important systems in drug delivery, especially for topical application,  but also orally and parenterally. The APV workshop on 01 to 02 April 2019 will provide a comprehensive insight, including formulation, characterization and processes.
but also orally and parenterally. The APV workshop on 01 to 02 April 2019 will provide a comprehensive insight, including formulation, characterization and processes.
Michael Herbig of RaDes will give a presentation on: ”Cream 2.0“ – Enhancing scope, performance and robustness of emulsions through rational design”. He is presenting new insights in distribution phenomena in emulsions and their effects on stability and performance, on effects of emulsifiers, consistency adjusting systems and approaches to modify skin penetration.
Find the program details here: https://www.apv-mainz.de/fileadmin/dateiablage/apv-mainz/Seminare/oeffentllich/6767_Emulsions_experts.pdf
If you’re interested in further information, don’t hesitate to contact us: info@rades-development.com.

With the implementation of the rheometer MCR 102 by Anton Paar GmbH we have added a new tool for formulation characterization to our equipment list. With this instrument we can perform both oscillatory and rotational measurements of semisolids and liquids. By variation of the chosen system (plate/plate, cone/plate, cylinder/beaker) we can obtain information on structure and behavior under stress of different formulation types.
Oscillation rheology allows prognosis for long-term stability and behavior under stress e.g. pumping into the storage vessel. Additionally, the influence of stress on the formulation can be measured. Using special methods, also the recovery of the structure after stress application can be shown. Does the formulation reach its structure before the stress application or is the formulation changed due to the destruction of the inner structure? Furthermore, the impact of varying concentrations, batches and suppliers of excipients can be compared to collect data on critical quality attributes e.g. the behavior under thermal stress.
Various formulations regarding type (emulsions, creams, ointments etc.), excipients (critical quality attributes) and storage conditions can be compared to evaluate the best possible prototype for your development and to support the trouble shooting of existing products.
If you have any questions, don’t hesitate to contact us: info@rades-development.com

Since end of October, a Waters ACQUITY™ UPLC™ H-Class PLUS with PDA eλ (UV/VIS) & QDa (single mass spectrometer) detectors expands the analytical equipment at RaDes GmbH.
We can cover a broad range of customer needs – from method development using Quality by Design, the transfer of analytical methods between HPLC and UPLC™ technology, up to the fast and reliable analysis of a large sample size.
What do these detectors stand for?
PDA eλ: highly sensitive photodiode array UV/VIS detector with an extended wavelength range between 190 nm to 800 nm to receive spectral information up to the visible range (e.g. to investigate discolorations) and for a robust quantification of compounds over a wide linear range.
QDa: Mass spectrometer (size of a UV/VIS detector) for peak tracking, quantification of compounds at a low concentration level or with a low UV absorption, assessment of co-elution.
The system contains next a solvent selection valve (six additional solvents) a column manager (four columns) to significantly save time and resources during method development.
All our HPLC and UPLC™ instruments are connected to the latest version of Empower™ 3 (Service Release 3) of Waters GmbH to ensure high-quality data management.
Particular benefits for our customers include:
1) Analysis of poor UV-absorbing molecules such as macrolides and peptides – a trend in dermatology.
2) Analysis of low dose formulations – another trend in dermatology.
3) Faster and better method development for classical HPLC:
- The method is developed using a DoE approach with a UPLC™. If necessary, co-eluting peaks are separated by mass spectrometry using the QDa detector and can therefore be precisely evaluated.
- The optimum conditions are selected from the design space considering resolution, run time and robustness.
- in silico transformation into a HPLC method using DoE.
- experimental confirmation on classical HPLC.
The preservation of creams and emulsions is often challenging. On the one hand, dermal drugs must be preserved in accordance with pharmacopoeia regulations in order to actively reduce microorganisms introduced. On the other hand, the number and concentration of the preservatives used should be kept as low as possible in order to optimize the compatibility of the formulation. Generally, depending on the product category and region (EU / USA), there are also maximum concentrations for the use of preservatives. Furthermore, a number of potential incompatibilities of preservatives with other formulation ingredients or primary packaging should be considered.
In the recently published study “Correlation of antimicrobial effects of phenoxyethanol with its free concentration in the water phase of o / w-emulsion gels.” (Puschmann et al., Eur J Pharm Biopharm (131) 152-161, 2018, www.sciencedirect.com) scientists from RaDes GmbH in collaboration with the Pharmaceutical Technology of the TU Braunschweig were able to gain new insights with high practical relevance for the optimization of preservation systems in emulsions.
We were able to establish a new method to accurately determine the concentration of the widely used phenoxyethanol preservative in the oil and water phases. In addition, it was possible to distinguish between free and micellar bound preservative within the water phase. As we expected, antimicrobial efficacy does not depend on the total concentration of the preservative but on the free concentration in the water phase. This in turn can be significantly influenced by the composition of the oil phase, by co-solvents in the water phase and by emulsifier type and concentration.
Our methods and insights make it possible to develop preservative systems faster and more robustly and to optimize the overall system in terms of antimicrobial performance, technical robustness and skin tolerance. Last but not least, based on this, the number of germ load tests necessary in the development phase can be purposefully reduced, thereby saving time and costs. This approach can also contribute significantly to the development of new, optimized preservatives.
